Rare Diseases
In the field of rare diseases, Ipsen takes a patient-focused approach to improving the lives of people affected by rare disorders and develops high-quality, innovative treatments that address unmet needs. Our goal is to support patients with drugs, services and solutions across the entire continuum of care, from diagnosis to treatment follow-up.
KEY FACTS
- In 2017, sales of our rare disease drugs totaled €75.1m, equivalent to 3.9% of our total sales
- A disease or disorder is defined as rare in Europe when it affects fewer than one in 2,000 people
- In the US, the definition applies to conditions that affect fewer than 200,000 people in the country at any given time
- An estimated 350+ million patients are suffering from one of over 7,000 rare diseases globally
- 50% of the people affected by rare diseases are children
- A total of 30% of rare disease patients die before the age of 5 and rare diseases are responsible for 35% of deaths in the first year of life
- Some 95% of rare diseases do not have a single FDA-approved drug treatment and as a result represents an area of high unmet medical need

We have a social contract with society; that means society is counting on us to accelerate the access of drugs to meet patients in need.
David Meek, CEO.
OUR RARE DISEASES STRATEGY
Ipsen has been active in the rare diseases field for many years and intends to bring new solutions to patients, notably children, in the future. Developing new treatments sooner, better, and faster for them is our goal. Yet, relatively common symptoms can hide underlying rare diseases leading to misdiagnosis and delayed treatment. We are focused on challenging this status quo with our dedicated focus on the discovery of new drugs and continued education about the signs and symptoms to aid early diagnosis. We understand that competing in the rare diseases space requires a distinct operating model and specific capabilities such as:
- Deep knowledge of the patient ecosystem
- Close collaboration with patient advocacy groups
- Efficient patient enrolement for clinical trials
- Extensive scientific expertise to interact with physicians who treat rare disease patients
- Ability to provide tailored patient services and education programs
We are proud to have developed these attributes at Ipsen and strongly believe that we will continue to add medical value for rare disease populations in the years to come.
OUR AREAS OF EXPERTISE
Somatuline® is used for the long-term treatment of acromegaly, a rare disease caused by excess growth hormone production as a result of a tumor in the pituitary gland, in patients who cannot be treated with surgery or radiation. Some 69,000 patients worldwide are affected by this disease. We are currently developing extended-release formulations of Somatuline® so that fewer injections would be required.
NutropinAq® is a liquid formulation of recombinant human growth hormone administered with the NutropinAq® Pen. Available in more than 20 countries, notably in Europe and Australia, it is indicated for the treatment of growth failure stemming from various origins.
Increlex® is a recombinant insulin-like growth factor (IGF-1) that treats growth delay in children. If IGF-1 is not present in sufficient quantities, the patient will not reach normal stature, despite having normal or high growth-hormone levels. As a result, these children do not respond adequately to growth hormone treatment.
Increlex® has been granted orphan drug status based on the low incidence of the disease, which affects fewer than five people per 10,000. It is the only drug available at the global level for this indication. In 2017, a new Ipsen manufacturing site was approved by both the EMA and FDA to produce Increlex®.
Ipsen also offers a number of other important solutions for patients with other debilitating or life-threatening conditions. For instance, Decapeptyl® (triptorelin) is also approved for the treatment of central precocious puberty (CPP) endometriosis, uterine fibroma, and in vitro fertilization. There is a potential opportunity for greater use of this treatment in the European Union, China and Russia.
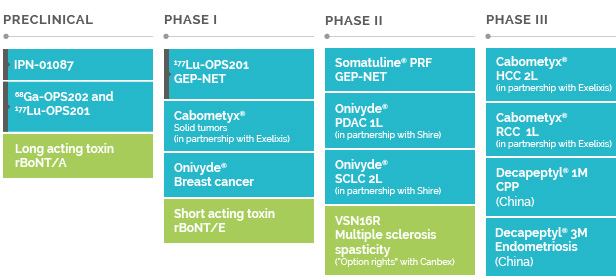
OUR RARE DISEASES PIPELINE
Further developing our presence in rare diseases constitutes a natural path forward for Ipsen. In the future, we will continue to expand in this area with high unmet medical need, leveraging expertise from development to commercialization to establish leadership positions and provide innovative treatments.
We will strengthen our pipeline and portfolio through targeted business development efforts, considering multiple factors in our assessment such as disease prevalence and severity, availability of treatments and the commercial and technological fit with Ipsen.
In particular, we will strengthen our portfolio with a particular emphasis on treatments for children. We believe we need to provide caregivers and physicians with the ability to treat these patients with new options and the launch of new trials is crucial this. This is why we believe the challenges are diminishing but the opportunities are growing.
€75.1 M
Sales in endocrinology in 2017
(3,9% of sales in 2017)