Neuroscience
Ipsen continues its long-standing commitment to a multi-modal approach to treating mobility impairment in adult and pediatric patients and improving their quality of life.
NEUROSCIENCE AT A GLANCE
Ipsen is one of the global leaders in the discovery, development, manufacture and commercialization of neurotoxins. Neuroscience is core to Ipsen’s specialty care business and is integral to the company’s overall strategy and long‐term growth.
Our long‐term commitment to advancing neuroscience and toxins research is evidenced by over 25 years of clinical experience. We are fully committed to improving people’s lives through safe and effective treatments, support and services in spasticity, movement disorders and specific indications in esthetic medicine.
KEY FACTS
- We have over 25 years of experience in neurotoxins
- In 2017, our neuroscience division achieved sales of €331.6m, equivalent to 17.4% of our income
- Our neuroscience team represents 21% of our Specialty Care business
- We have over 1000 employees dedicated to neuroscience
- In 2017, our ambition for neurotoxins was boosted by a £22 million investment at our campus at Wrexham, U.K.
- We are the only company with recombinant toxins in our portfolio
- With a single product, Dysport®, we are able to treat a range of therapeutic indications including cervical dystonia, adult spasticity, hyperhidrosis, blepharospasm, hemifacial spasm and pediatric lower limb spasticity
- Dysport® is authorized in more than 85 countries for seven therapeutic and two esthetic indications
- Dysport® has 5 million treatment years of patient experience and is scientifically supported by over 1,300 peer‐reviewed publications
- We have a further 400 patents in our neuroscience division, most of which came from internal research activities
- Our partnership with Galderma, which has been extended until 2036, means that together we represent 75% of the world market for neurotoxins Through our 9 Ixcellence Network centers we have trained 728 physicians in the last five years.

Patients are at the heart of our strategy and leading our drive for innovation in patient care.
David Meek, CEO.
OUR NEUROSCIENCE STRATEGY
Neuroscience is advancing at an unprecedented rate and we are proud to be at the forefront of this transformation. By doing so, we hope to help patients take back control of their lives.
We are one of a handful of companies who specialise in botulinum toxins and we are constantly striving to do everything we can to help relieve the debilitating and painful symptoms of spasticity in adults and children. We are the only company to have recombinant neurotoxins in development. This is important because the application of recombinant techniques to create novel botulinum toxin-based medicines with different onset and duration of action, gives clinicians the ability to choose the most appropriate neurotoxin for the patient.
In 2017, the first recombinant neurotoxin was administered to a human – a major milestone for our fast-onset and short-acting toxin type E – and we are now progressing into the development program. This was a hugely important milestone because the sooner the therapist can add the botulinum neurotoxin to the rehabilitation program, the better the outcome for the patient — including reducing the severity of spasticity over time.
We have worked tirelessly to provide access to Dysport® in more than 85 countries for two esthetic indications and seven therapeutic indications including adult and paediatric spasticity and cervical dystonia. With Dysport®, we are able to offer a single product to treat a range of therapeutic indications. It is also the first and only botulinum toxin approved by the FDA for the treatment of upper and lower limb spasticity in adults, as well as for the treatment of lower limb spasticity in children aged two and older. In 2017 alone, 36 countries launched new Dysport® indications.
Our strong partnership with Galderma on Dysport® means that together we now cover three-quarters of the world market for neurotoxins in esthetic indications. As a function of the partnership, Ipsen predominantly promotes therapeutic indications for Dysport®, while Galderma distributes esthetic treatment under the brand names of Dysport® and Azzalure®. Ipsen and Galderma will also collaborate on the future development and commercialization of new neurotoxin therapies. The partnership with Galderma now includes the United States, Canada, Europe, Brazil, Australia and some Asia-Pacific countries.
Going forward, we are focused on expanding into new markets, growing our presence in North America, extending the range of clinical indications for Dysport® and building a sustainable pipeline of novel recombinant toxins to improve the lives of patients with severely debilitating conditions.
OUR AREAS OF EXPERTISE
Neurotoxins
Since 1990, Ipsen has focused on pioneering research in neurotoxins and more recently on recombinant neurotoxin engineering. We are at the forefront of the field in this technology – and with this leadership position comes responsibilities, the first of which is to relentlessly search for new neurotoxins that could improve patient outcomes and address unmet medical needs.
Neurotoxins (such as Dysport®) are important biological drugs for treating patients with musculoskeletal or smooth muscle disorders following neurological disease. We have achieved multiple global approvals of Dysport® in the last four years in adults (e.g. following stroke, traumatic brain injury or multiple sclerosis) and children with spasticity, which demonstrates our commitment to these fields.
Our drug treatment, Dysport®, is based on the type-A botulinum toxin, which inhibits the transmission of nerve impulses to the muscle. Botulinum toxin injections cause contracted muscles to relax, relieving patients’ symptoms and helping to improve the quality of their daily lives. Dysport® was first registered for the treatment of blepharospasm in the United Kingdom in 1990 and has been marketed since 1991.
Today, Dysport® is used primarily for patients with spasticity, cervical dystonia, hemifacial spasm, blepharospasm and hyperhidrosis. In esthetic medicine, Dysport® is indicated for the reduction of glabellar lines (frown lines). The recent approval in the United States of Dysport® for the treatment of upper limb spasticity in adults and lower limb spasticity in children aged two and older was a strong endorsement of our clinical trials.
In esthetic medicine, Ipsen has been granted the right to distribute Dysport® in esthetic medicine to Galderma, a pharmaceutical company specializing in the research, development and marketing of dermatological treatments. As a function of the partnership, Ipsen predominantly promotes therapeutic indications for Dysport®, while Galderma distributes esthetic treatment under the brand names of Dysport® and Azzalure®. Ipsen and Galderma will also collaborate on the future development and commercialization of new neurotoxin therapies.
Next-generation neurotoxins
Botulinum toxins have the potential for broad applications across multiple therapeutic areas, including urology, neurology, oncology, endocrinology and regenerative medicine. We are planning for the future with research on new recombinant botulinum toxins and on the promising area of targeted secretion inhibition.
Collaborative partnerships with renowned university research centers are ongoing and at the center of finding new ways to use our neurotoxin pipeline to combat debilitating conditions that burden the lives of patients.
Research partnerships continue with Harvard University, while a new research partnership with the IMCB in Singapore, under the aegis of the Agency for Science, Technology and Research (A*STAR) , aims to advance understanding of botulinum neurotoxin biology.
Commitment to spasticity in multiple sclerosis
Ipsen and GW Pharmaceuticals have an agreement giving Ipsen promotion and distribution rights in Latin America for Mevatyl®, an oromucosal spray indicated as treatment for symptom improvement in adult patients with moderate to severe spasticity due to multiple sclerosis. GW Pharmaceuticals and Ipsen have gained approval in Brazil and are conducting regulatory filings in other selected countries in Latin America for this indication.
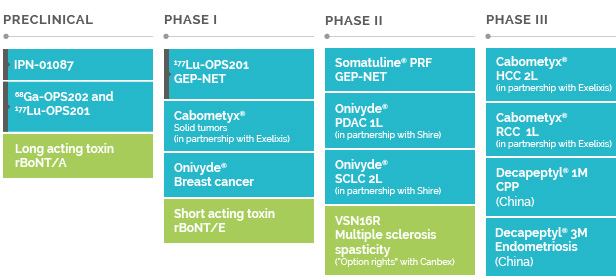
OUR NEUROSCIENCE PIPELINE
At our R&D centers around the world, and through numerous collaborative projects and partnerships with top academic institutes and investigators, we are pioneering research into neurotoxins. We have a leading-edge protein engineering expertise in toxins and we are pushing the boundaries of neurotoxin research to provide innovative therapeutic and esthetic solutions to help patients take back control of their lives.
Our research team is distinguished by the brightest minds in recombinant technologies fuelled by a passion for advancing neurotoxin research for patient benefit. Our world class R&D centres (Oxford, UK & Les Ulis, France) and manufacturing centre (Wrexham, UK) are pushing the technological boundaries to develop the next‐generation of recombinant toxins that will target an even broader spectrum of conditions. Our ambition in neurotoxins was boosted in 2017 by a £22 million investment in our Wrexham campus and the approval of two new state‐of‐the‐art facilities, Unit 12 in Wrexham and the Bioprocessing Suite. These biologics facilities will accelerate the production of novel toxins to support research as well as increase manufacturing capacity to meet the needs of today’s patients.
Further clinical trials are ongoing to generate additional data in support of therapeutic uses of Dysport®. For example, we aim to explore and develop additional Dysport® indications in neurology and urology. Research is also underway on the development of a liquid formulation for both esthetic and Therapeutic uses. We are also investing in Dysport® to support two new indications on top of our Dysport® phase III programs.
We are committed to continuing a tradition of delivering high-level scientific evidence with current post-marketing studies such as the On-Time pilot study, focused on early treatment with Dysport® in stroke patients, and the Upper Limb International Spasticity (ULIS) study, involving 1,000 people in 14 countries. A newly launched ENGAGE study was launched in 2016 to assess the effect of abobotulinumtoxinA administered simultaneously in upper and lower limbs in conjunction with a guided self-rehabilitation contract in adult patients with spastic hemiparesis.
Ipsen is also one of the very few organizations to master the manufacture and development of targeted secretion inhibitors (TSIs), a new class of molecule in which case the toxin-derived secretion inhibitor is directed towards different types of cells depending on the cellular targeting strategy used.
Research partnerships enable us to discover new molecules, enhance the potency, efficacy and formulation of existing treatments and commercialise our products. All our partnerships are helping to transform our scientific know‐how into cutting‐edge therapies that we believe will improve many more lives in the future.
We will continue pushing the boundaries of science to unlock the potential of neurotoxins by identifying new applications for existing toxins and developing a portfolio of novel recombinant toxins. We have the vision, the talent and the determination to lead the way in this new era of neurotoxin research and we look forward to unveiling new breakthroughs in the future.
WORKING WITH PATIENTS AND HEALTHCARE PROVIDERS
Ipsen has built strong, long-term partnerships with a number of patient associations and healthcare providers, including:
- Dystonia Europe, an organization representing dystonia patients across the continent, and also with the American Dystonia Society. We continue to support initiatives for physicians who seek further research in cervical dystonia, disease awareness campaigns for patients and the creation of patient networks in Europe.
- Ipsen’s I-CAN program is an innovative spasticity management program that engages patients in their own treatment.
- Our long-running “Ixcellence Network” trains physicians in more than 20 countries and helps them set goals for self-guided rehabilitation. For esthetics practitioners, our educational masterclasses increase clinical and practical expertise for better outcomes
- We are proud sponsors of the David Marsden’s Award which is presented every two years to encourage and drive research on dystonia, especially by young scientists in Europe.
- We sponsored the Burden of Stroke in Europe report, which was launched in the EU parliament in May 2017 and which highlighted disparities between countries along the stroke care pathway.
- Our C.L.I.M.B.® (Continuum of Learning to Improve Management with Botulinum Toxin) programme is a multi‐tiered learning continuum designed to help physicians improve their clinical skills involving the appropriate use of Dysport® for the treatment of adults with cervical dystonia and spasticity.
€331.6 M
Sales in neuroscience in 2017
(17,4% of sales in 2017)
25 years
Expertise in neurotoxins
> 200
Patents in neurology